Preclinical CRO Market Demand, Size, Share, Industry Growth Report 2024

IMARC Group, a leading market research company, has recently released a report titled “Preclinical CRO Market Report by Service (Bioanalysis and DMPK Studies, Toxicology Testing, and Others), End Use (Biopharmaceutical Companies, Government and Academic Institutes, Medical Device Companies), and Region 2024-2032”. The study provides a detailed analysis of the industry, including the preclinical CRO market share, trends, size, and industry trends forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
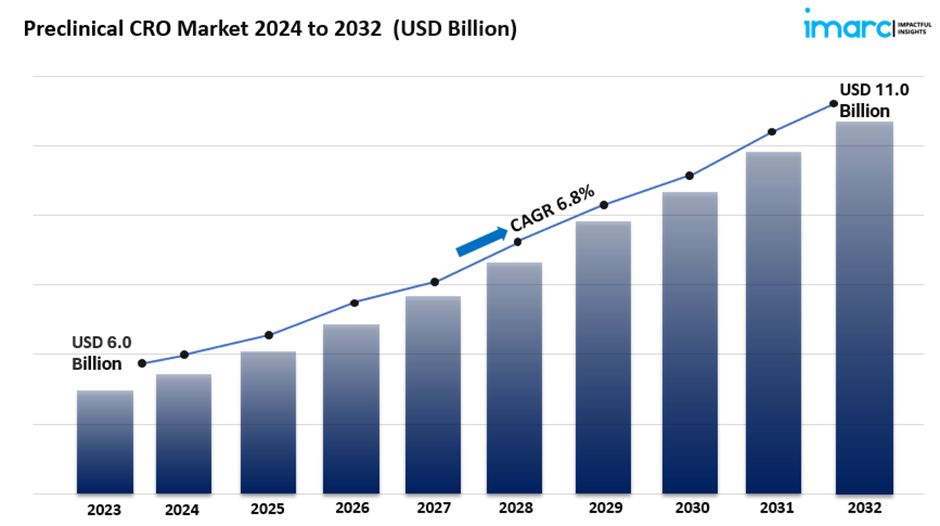
The global preclinical CRO market size reached US$ 6.0 Billion in 2023. Looking forward, IMARC Group expects the market to reach US$ 11.0 Billion by 2032, exhibiting a growth rate (CAGR) of 6.8% during 2024-2032.
Global Preclinical CRO Market Trends:
The increasing focus on personalized medicine, which demands more tailored preclinical studies to evaluate drug efficacy and safety in specific genetic populations, is creating a positive outlook for the market growth.
Moreover, the burgeoning integration of digital tools, such as electronic data capture (EDC) systems and cloud-based platforms, that enhance data management and collaboration capabilities in preclinical studies is fostering the market growth.
Furthermore, the increasing emphasis on sustainability and reducing the environmental impact of research, encouraging CROs to innovate with eco-friendly practices, such as reducing animal usage and minimizing waste, is boosting the market growth.
Request to Get the Sample Report:
https://www.imarcgroup.com/preclinical-cro-market/requestsample
Factors Affecting the Growth of the Preclinical CRO Industry:
· Increasing Drug Development Activities:
The rise in drug development activities across the globe is creating a positive outlook for the market growth.
Moreover, the increasing pressure on pharmaceutical companies to innovate and bring new drugs to market to address unmet medical needs and stay ahead in a competitive landscape is fostering the market growth. Besides this, the growing prevalence of chronic diseases, the geriatric population, and advances in biotechnology, which demand rapid discovery and preclinical testing of new therapeutic agents, are favoring the market growth.
Along with this, the heightened adoption of contract research organizations (CROs) as they provide a range of services, including toxicology studies, pharmacokinetics, and pharmacodynamics assessments, which are essential for determining the safety and efficacy of new drugs before they enter clinical trials, is enhancing the market growth.
· Rapid Technological Advancements in Preclinical Research:
The increasing adoption of cutting-edge technologies, such as high-throughput screening, clustered regularly interspaced short palindromic repeats (CRISPR) gene editing, artificial intelligence (AI), and advanced imaging techniques that revolutionize preclinical research enabling more accurate and faster drug candidate assessments is catalyzing the market growth. These technologies enhance the ability to predict human responses to new drugs more accurately, reduce the risk of late-stage failures, and optimize the drug development timeline.
Moreover, the rising investment by CROs in advanced technologies to offer more comprehensive and sophisticated services to their clients, enhancing their appeal as strategic partners in drug development, is stimulating the market growth.
· Growing Regulatory Pressure and Need for Compliance:
The introduction of stringent regulatory requirements for drug approval, prompting pharmaceutical companies to enhance their focus on compliance, is driving the market growth.
Moreover, the growing need for compliance during drug development, particularly in the preclinical phase, which includes comprehensive safety and efficacy assessments, is boosting the market growth. Along with this, the growing adoption of preclinical CROs as they specialize in navigating these complex regulatory landscapes, ensuring that studies are conducted in compliance with all necessary standards and guidelines, is fueling the market growth.
Moreover, the increasing scrutiny from regulatory bodies concerning the ethical use of animals in research and the accuracy of preclinical data, compelling companies to outsource to CROs, is fostering the market growth.
Preclinical CRO Market Report Segmentation:
By Service:
· Bioanalysis and DMPK Studies
· Toxicology Testing
· Others
Toxicology testing accounted for the largest market share as it is essential for ensuring the safety of drug candidates before clinical trials, making it a critical component of preclinical studies.
By End Use:
· Biopharmaceutical Companies
· Government and Academic Institutes
· Medical Device Companies
Biopharmaceutical companies represented the largest segment as they rely on preclinical CROs to streamline drug development processes and reduce costs.
Regional Insights:
· North America
· Asia-Pacific
· Europe
· Latin America
· Middle East and Africa
North America's dominance in the preclinical CRO market is attributed to its advanced healthcare infrastructure, high research, and development (R&D) spending, and the presence of numerous leading pharmaceutical and biotechnology companies.
Competitive Landscape with Key Players:
The competitive landscape of the preclinical CRO market size has been studied in the report with the detailed profiles of the key players operating in the market.
Some of These Key Players Include:
· Charles River Laboratories Inc.
· Covance Inc. (Laboratory Corporation of America Holdings)
· Eurofins Scientific
· ICON Plc
· MD Biosciences Inc. (MLM Medical Labs)
· Medpace
· Parexel International Corporation
· PPD Inc.
· Wuxi AppTec
Ask Analyst for Customized Report:
https://www.imarcgroup.com/request?type=report&id=3715&flag=C
Key Highlights of the Report:
· Market Performance (2018-2023)
· Market Outlook (2024-2032)
· Market Trends
· Market Drivers and Success Factors
· Impact of COVID-19
· Value Chain Analysis
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact Us:
IMARC Group
134 N 4th St
Brooklyn, NY 11249, USA
Website: imarcgroup.com
Email: sales@imarcgroup.com
Americas: +1-631-791-1145 | Europe & Africa: +44-753-713-2163 | Asia: +91-120-433-0800
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Παιχνίδια
- Gardening
- Health
- Κεντρική Σελίδα
- Literature
- Music
- Networking
- άλλο
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness




