Blood Pressure Monitoring Devices Market Report: Unleashing Growth Prospects and Overcoming Challenges
United States of America –The Insight Partners is delighted to announce the release of its latest research report, "Blood Pressure Monitoring Devices Market: Global Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2022–2030". This detailed report presents a thorough analysis of the emerging market scenario for blood pressure monitoring devices, highlighting innovation, demand patterns, and investment prospects.
Overview of Market
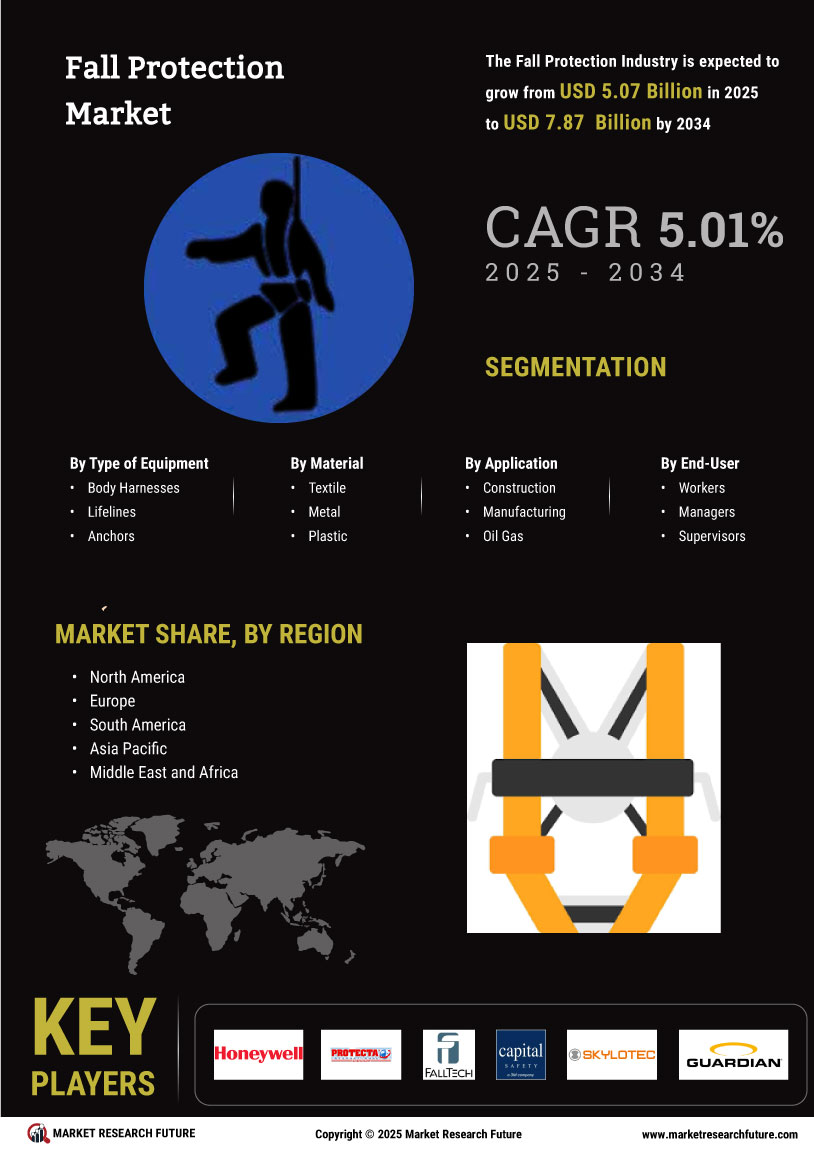
The market for blood pressure monitoring devices has witnessed tremendous growth, fueled by improving cardiovascular health consciousness, growing cases of hypertension, and the embracement of remote patient monitoring technology. In this report, the evolving dynamics of the market are analyzed and strategic insights into how technology, consumer behavior, and regulatory trends are influencing the future of blood pressure monitoring solutions across the world.
Key Findings and Insights
Market Size and Growth
Historical Data: The size of the blood pressure monitoring devices market is expected to increase from US$ 2,387.21 million in 2022 to US$ 5,346.65 million in 2030; the market is expected to register a CAGR of 10.6% between 2022 and 2030.
Chief Growth Drivers:
Increased incidence of lifestyle disorders like hypertension and obesity
Increase in geriatric population with chances of cardiovascular diseases
Increase in home healthcare and telemedicine services
Growing demand for wearable and portable health monitoring devices
Market Segmentation
By Product
Mercury Columns
Aneroid Blood Pressure Monitors
Digital Blood Pressure Monitors
By Type
Arm
Wrist
Finger
By End User
Hospitals & Clinics
Homecare Settings
Ambulatory Surgical Centers
Get Sample PDF Guide: https://www.theinsightpartners.com/Sample/TIPMD00002666
Seeing Emerging Trends
Technological Breakthroughs:
Use of Bluetooth and Wi-Fi in digital monitors for real-time monitoring
Creation of cuffless and wearable monitoring devices
Application of AI to predictive analytics and hypertension management for individual patients
Shifting Consumer Preferences:
Migration towards blood pressure monitoring at home for ease and constant monitoring
Growing demand from consumers for small, easy-to-use, and cost-effective devices
Regulatory Changes:
FDA clearances for new monitoring devices
Government programs to enhance cardiovascular diagnostic access
Reforms in insurance policies favoring remote health monitoring and diagnostics
Growth Opportunities
The blood pressure monitoring devices market offers several high-growth opportunities:
Growth in emerging markets with growing healthcare awareness and infrastructure
Medical device companies partnering with technology companies for intelligent monitoring devices
Inclusion in digital health platforms for managing chronic disease
Establishment of non-invasive and wearable blood pressure technologies
Conclusion
The Blood Pressure Monitoring Devices Market: Global Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2022–2030 is a strategic document for players wishing to enter or increase their foothold in the fast-growing health technology market. Based on current trends, market segmentation, competitive profile, and future outlook, the report equips decision-makers with the tools required for effective and well-informed business strategies.
About The Insight Partners
The Insight Partners is among the leading market research and consulting firms in the world. We take pride in delivering exclusive reports along with sophisticated strategic and tactical insights into the industry. Reports are generated through a combination of primary and secondary research, solely aimed at giving our clientele a knowledge-based insight into the market and domain. This is done to assist clients in making wiser business decisions. A holistic perspective in every study undertaken forms an integral part of our research methodology and makes the report unique and reliable.
United States of America –The Insight Partners is delighted to announce the release of its latest research report, "Blood Pressure Monitoring Devices Market: Global Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2022–2030". This detailed report presents a thorough analysis of the emerging market scenario for blood pressure monitoring devices, highlighting innovation, demand patterns, and investment prospects.
Overview of Market
The market for blood pressure monitoring devices has witnessed tremendous growth, fueled by improving cardiovascular health consciousness, growing cases of hypertension, and the embracement of remote patient monitoring technology. In this report, the evolving dynamics of the market are analyzed and strategic insights into how technology, consumer behavior, and regulatory trends are influencing the future of blood pressure monitoring solutions across the world.
Key Findings and Insights
Market Size and Growth
Historical Data: The size of the blood pressure monitoring devices market is expected to increase from US$ 2,387.21 million in 2022 to US$ 5,346.65 million in 2030; the market is expected to register a CAGR of 10.6% between 2022 and 2030.
Chief Growth Drivers:
Increased incidence of lifestyle disorders like hypertension and obesity
Increase in geriatric population with chances of cardiovascular diseases
Increase in home healthcare and telemedicine services
Growing demand for wearable and portable health monitoring devices
Market Segmentation
By Product
Mercury Columns
Aneroid Blood Pressure Monitors
Digital Blood Pressure Monitors
By Type
Arm
Wrist
Finger
By End User
Hospitals & Clinics
Homecare Settings
Ambulatory Surgical Centers
Get Sample PDF Guide: https://www.theinsightpartners.com/Sample/TIPMD00002666
Seeing Emerging Trends
Technological Breakthroughs:
Use of Bluetooth and Wi-Fi in digital monitors for real-time monitoring
Creation of cuffless and wearable monitoring devices
Application of AI to predictive analytics and hypertension management for individual patients
Shifting Consumer Preferences:
Migration towards blood pressure monitoring at home for ease and constant monitoring
Growing demand from consumers for small, easy-to-use, and cost-effective devices
Regulatory Changes:
FDA clearances for new monitoring devices
Government programs to enhance cardiovascular diagnostic access
Reforms in insurance policies favoring remote health monitoring and diagnostics
Growth Opportunities
The blood pressure monitoring devices market offers several high-growth opportunities:
Growth in emerging markets with growing healthcare awareness and infrastructure
Medical device companies partnering with technology companies for intelligent monitoring devices
Inclusion in digital health platforms for managing chronic disease
Establishment of non-invasive and wearable blood pressure technologies
Conclusion
The Blood Pressure Monitoring Devices Market: Global Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2022–2030 is a strategic document for players wishing to enter or increase their foothold in the fast-growing health technology market. Based on current trends, market segmentation, competitive profile, and future outlook, the report equips decision-makers with the tools required for effective and well-informed business strategies.
About The Insight Partners
The Insight Partners is among the leading market research and consulting firms in the world. We take pride in delivering exclusive reports along with sophisticated strategic and tactical insights into the industry. Reports are generated through a combination of primary and secondary research, solely aimed at giving our clientele a knowledge-based insight into the market and domain. This is done to assist clients in making wiser business decisions. A holistic perspective in every study undertaken forms an integral part of our research methodology and makes the report unique and reliable.
Blood Pressure Monitoring Devices Market Report: Unleashing Growth Prospects and Overcoming Challenges
United States of America –The Insight Partners is delighted to announce the release of its latest research report, "Blood Pressure Monitoring Devices Market: Global Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2022–2030". This detailed report presents a thorough analysis of the emerging market scenario for blood pressure monitoring devices, highlighting innovation, demand patterns, and investment prospects.
Overview of Market
The market for blood pressure monitoring devices has witnessed tremendous growth, fueled by improving cardiovascular health consciousness, growing cases of hypertension, and the embracement of remote patient monitoring technology. In this report, the evolving dynamics of the market are analyzed and strategic insights into how technology, consumer behavior, and regulatory trends are influencing the future of blood pressure monitoring solutions across the world.
Key Findings and Insights
Market Size and Growth
Historical Data: The size of the blood pressure monitoring devices market is expected to increase from US$ 2,387.21 million in 2022 to US$ 5,346.65 million in 2030; the market is expected to register a CAGR of 10.6% between 2022 and 2030.
Chief Growth Drivers:
Increased incidence of lifestyle disorders like hypertension and obesity
Increase in geriatric population with chances of cardiovascular diseases
Increase in home healthcare and telemedicine services
Growing demand for wearable and portable health monitoring devices
Market Segmentation
By Product
Mercury Columns
Aneroid Blood Pressure Monitors
Digital Blood Pressure Monitors
By Type
Arm
Wrist
Finger
By End User
Hospitals & Clinics
Homecare Settings
Ambulatory Surgical Centers
Get Sample PDF Guide: https://www.theinsightpartners.com/Sample/TIPMD00002666
Seeing Emerging Trends
Technological Breakthroughs:
Use of Bluetooth and Wi-Fi in digital monitors for real-time monitoring
Creation of cuffless and wearable monitoring devices
Application of AI to predictive analytics and hypertension management for individual patients
Shifting Consumer Preferences:
Migration towards blood pressure monitoring at home for ease and constant monitoring
Growing demand from consumers for small, easy-to-use, and cost-effective devices
Regulatory Changes:
FDA clearances for new monitoring devices
Government programs to enhance cardiovascular diagnostic access
Reforms in insurance policies favoring remote health monitoring and diagnostics
Growth Opportunities
The blood pressure monitoring devices market offers several high-growth opportunities:
Growth in emerging markets with growing healthcare awareness and infrastructure
Medical device companies partnering with technology companies for intelligent monitoring devices
Inclusion in digital health platforms for managing chronic disease
Establishment of non-invasive and wearable blood pressure technologies
Conclusion
The Blood Pressure Monitoring Devices Market: Global Industry Trends, Share, Size, Growth, Opportunity, and Forecast 2022–2030 is a strategic document for players wishing to enter or increase their foothold in the fast-growing health technology market. Based on current trends, market segmentation, competitive profile, and future outlook, the report equips decision-makers with the tools required for effective and well-informed business strategies.
About The Insight Partners
The Insight Partners is among the leading market research and consulting firms in the world. We take pride in delivering exclusive reports along with sophisticated strategic and tactical insights into the industry. Reports are generated through a combination of primary and secondary research, solely aimed at giving our clientele a knowledge-based insight into the market and domain. This is done to assist clients in making wiser business decisions. A holistic perspective in every study undertaken forms an integral part of our research methodology and makes the report unique and reliable.
0 Commentarii
0 Distribuiri
5K Views
0 previzualizare